What began a dozen years ago as a conversation between a PhD in bioengineering at the University of Illinois and a sleep specialist at Carle Foundation Hospital is now an FDA-cleared wearable product designed to make it easier to diagnose obstructive sleep apnea. Now the pair is in the process of seeking a manufacturer for the at-home sensor.
Charles Davies, MD, PhD, at Carle Foundation Hospital, first met John Rogers, PhD, at the University of Illinois thanks to a tip from his wife, Professor Lori Raetzman, a university physiologist. She mentioned a presentation on campus featuring Rogers and the subject of wearables. Davies not only attended but also approached Rogers after the talk about creating wearables for a sleep lab. That started the research rolling.
thanks to a tip from his wife, Professor Lori Raetzman, a university physiologist. She mentioned a presentation on campus featuring Rogers and the subject of wearables. Davies not only attended but also approached Rogers after the talk about creating wearables for a sleep lab. That started the research rolling.
The two men collaborated with others on a study of the ANNE (advanced neo-natal epidermal) sleep system. Rogers left for the Northwestern University McCormick School of Engineering but the collaboration continued with over 70 individuals at Carle and 150 at Northwestern sleep labs wearing the flexible dual sensor system that is easily mounted on the chest and finger. The findings showed it is 90% effective and the team sent data to the FDA to secure clearance to use the sensor.
“We know obstructive sleep apnea (OSA, where breathing repeatedly stop and starts) increases the risk for strokes, heart attacks, metabolic disorders and motor vehicle accidents,” Dr. Davies said. “Up to one in four people in the U.S. have OSA and most are undiagnosed.”
Currently, the gold standard for diagnosis is a polysomnogram, ideally performed in an accredited sleep center. An important difference between using the device at home and the traditional sleep center evaluation is brain waves are also measured during the evaluation at a sleep center. However, COVID-19 raised new concerns and sleep labs reduced capacity to manage risk of exposure to patients and staff. Plus, a patient who comes in to the lab for the testing may find it difficult to sleep in unfamiliar surroundings.
The ANNE sensor could provide a home-based option to not only minimize COVID-19 exposure, but also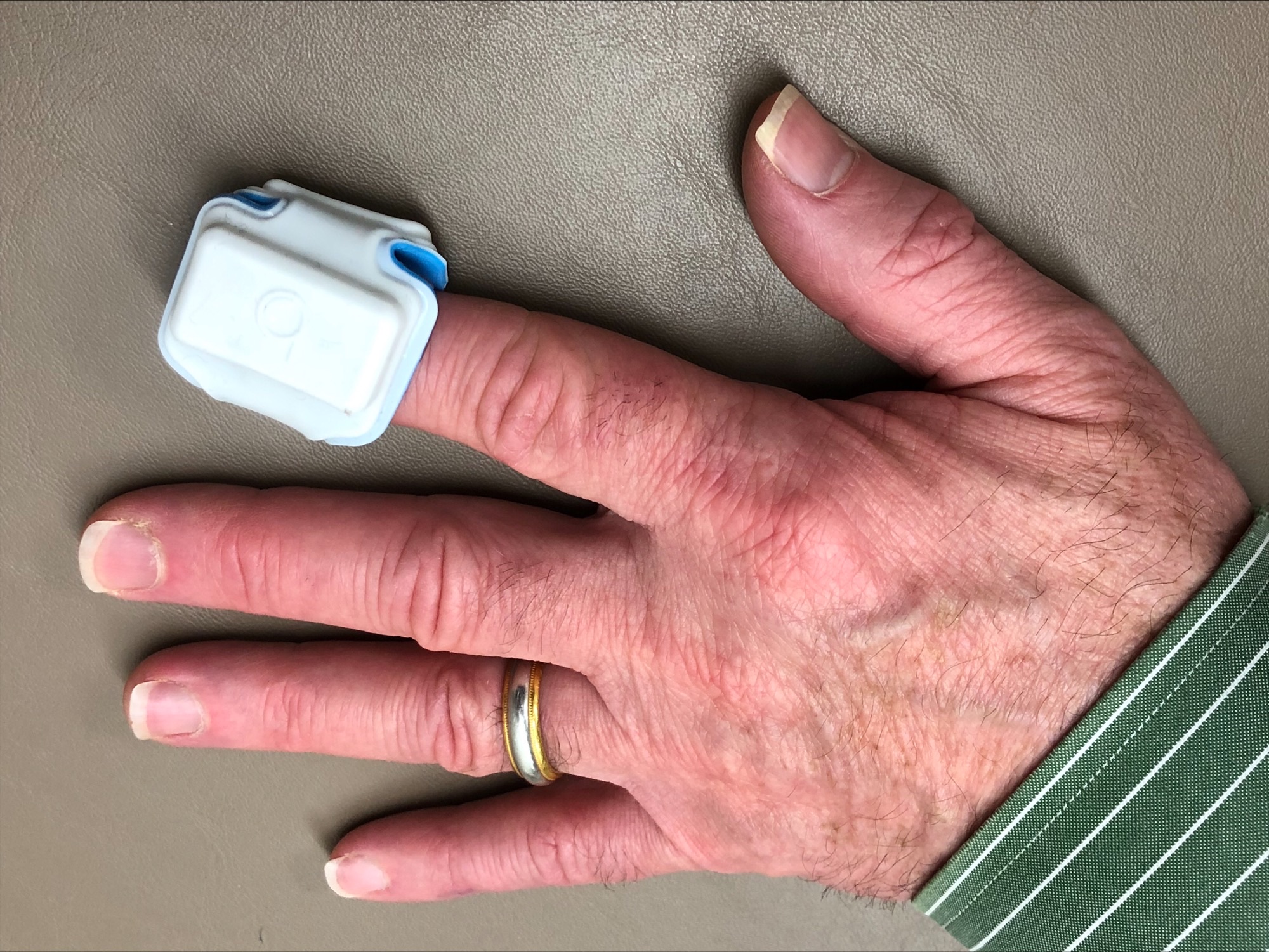 reduce diagnostic costs and allow the patient a more familiar sleep environment. Electrical circuits inside the wearable device detect movement and there is a transistor that a smart phone can pick up. The following morning, study facilitators send the entire system, which weighs a few ounces, through the mail, Dr. Davies said. The device includes an easy-to-clean covering and the hope is the device will potentially lower the cost of the testing.
reduce diagnostic costs and allow the patient a more familiar sleep environment. Electrical circuits inside the wearable device detect movement and there is a transistor that a smart phone can pick up. The following morning, study facilitators send the entire system, which weighs a few ounces, through the mail, Dr. Davies said. The device includes an easy-to-clean covering and the hope is the device will potentially lower the cost of the testing.

“The key is to scale up production,” Dr. Davies said.
If a patient has many symptoms of obstructive sleep apnea and no other health conditions requiring a sleep lab study, then they can take a home study with ANNE, Dr. Davies said. That could be as many as 25% of the undiagnosed patients or approximately 4 million people in the U.S. he said. If clinicians note some signs and symptoms of obstructive sleep apnea, then the patient would complete the traditional sleep center evaluation.
There is currently at-home equipment that can diagnose obstructive sleep apnea, but it is bulky and just not as accurate as the ANNE sensor, Dr. Davies said.
To learn more about the ANNE sleep system, go to https://www.sibelhealth.com/.
Charles Davies, MD, PhD, at Carle Foundation Hospital, first met John Rogers, PhD, at the University of Illinois
 thanks to a tip from his wife, Professor Lori Raetzman, a university physiologist. She mentioned a presentation on campus featuring Rogers and the subject of wearables. Davies not only attended but also approached Rogers after the talk about creating wearables for a sleep lab. That started the research rolling.
thanks to a tip from his wife, Professor Lori Raetzman, a university physiologist. She mentioned a presentation on campus featuring Rogers and the subject of wearables. Davies not only attended but also approached Rogers after the talk about creating wearables for a sleep lab. That started the research rolling.The two men collaborated with others on a study of the ANNE (advanced neo-natal epidermal) sleep system. Rogers left for the Northwestern University McCormick School of Engineering but the collaboration continued with over 70 individuals at Carle and 150 at Northwestern sleep labs wearing the flexible dual sensor system that is easily mounted on the chest and finger. The findings showed it is 90% effective and the team sent data to the FDA to secure clearance to use the sensor.
“We know obstructive sleep apnea (OSA, where breathing repeatedly stop and starts) increases the risk for strokes, heart attacks, metabolic disorders and motor vehicle accidents,” Dr. Davies said. “Up to one in four people in the U.S. have OSA and most are undiagnosed.”
Currently, the gold standard for diagnosis is a polysomnogram, ideally performed in an accredited sleep center. An important difference between using the device at home and the traditional sleep center evaluation is brain waves are also measured during the evaluation at a sleep center. However, COVID-19 raised new concerns and sleep labs reduced capacity to manage risk of exposure to patients and staff. Plus, a patient who comes in to the lab for the testing may find it difficult to sleep in unfamiliar surroundings.
The ANNE sensor could provide a home-based option to not only minimize COVID-19 exposure, but also
 reduce diagnostic costs and allow the patient a more familiar sleep environment. Electrical circuits inside the wearable device detect movement and there is a transistor that a smart phone can pick up. The following morning, study facilitators send the entire system, which weighs a few ounces, through the mail, Dr. Davies said. The device includes an easy-to-clean covering and the hope is the device will potentially lower the cost of the testing.
reduce diagnostic costs and allow the patient a more familiar sleep environment. Electrical circuits inside the wearable device detect movement and there is a transistor that a smart phone can pick up. The following morning, study facilitators send the entire system, which weighs a few ounces, through the mail, Dr. Davies said. The device includes an easy-to-clean covering and the hope is the device will potentially lower the cost of the testing.
“The key is to scale up production,” Dr. Davies said.
If a patient has many symptoms of obstructive sleep apnea and no other health conditions requiring a sleep lab study, then they can take a home study with ANNE, Dr. Davies said. That could be as many as 25% of the undiagnosed patients or approximately 4 million people in the U.S. he said. If clinicians note some signs and symptoms of obstructive sleep apnea, then the patient would complete the traditional sleep center evaluation.
There is currently at-home equipment that can diagnose obstructive sleep apnea, but it is bulky and just not as accurate as the ANNE sensor, Dr. Davies said.
To learn more about the ANNE sleep system, go to https://www.sibelhealth.com/.
Categories: Redefining Healthcare, Community
Tags: Champaign-Urbana, research, sleep
